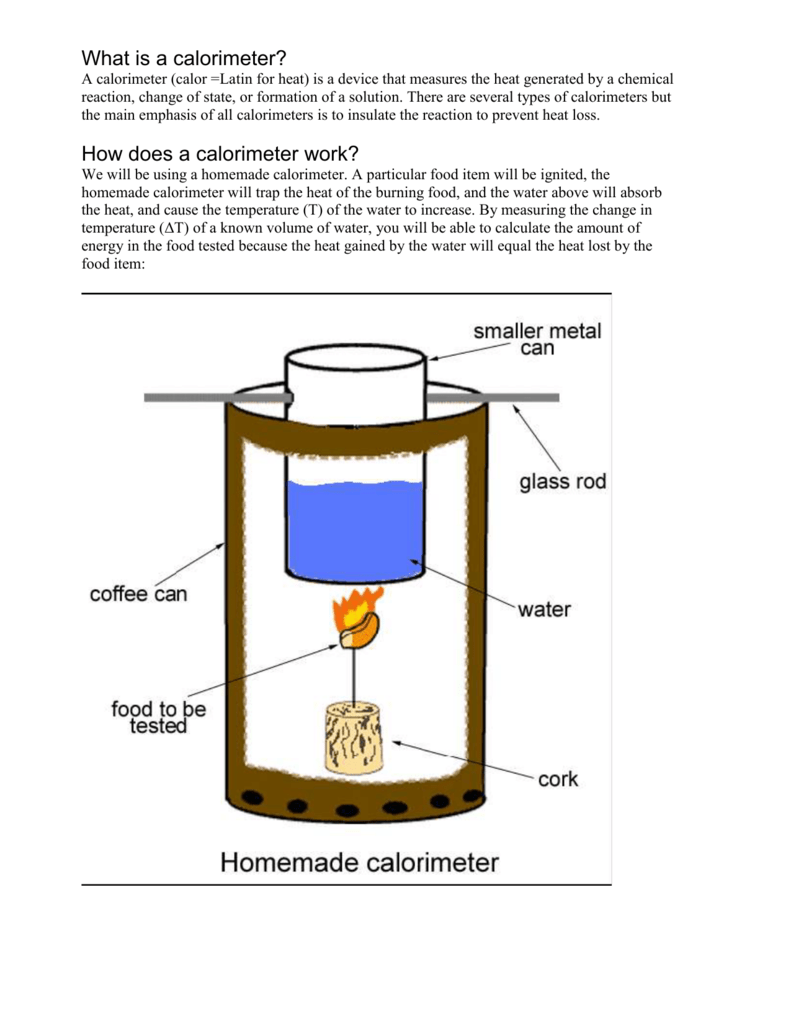
Food Calorimetry Lab Procedure . try this class experiment to investigate how much energy different foods contain. The bomb calorimeter is set up as shown in fig.1 above. food calorimetry allows us to determine the number of calories per gram of food. The caloric content of a food item will be determined using “soda can” calorimetry, where the heat lost through. in the laboratory, you can determine energy content by burning a portion of food and capturing the heat released to a known. in order to obtain caloric content in food, food scientists perform an indirect measure of the amount of heat generated from the burning or. Prepare the sample in the. Determine the number of kilocalories (food calories) released by the burning food sample. In this practical, students burn a sample of a foodstuff of known mass,. In this activity, you will burn a piece of food and. prepare a table of your results.
from studylib.net
Determine the number of kilocalories (food calories) released by the burning food sample. try this class experiment to investigate how much energy different foods contain. In this activity, you will burn a piece of food and. food calorimetry allows us to determine the number of calories per gram of food. in order to obtain caloric content in food, food scientists perform an indirect measure of the amount of heat generated from the burning or. In this practical, students burn a sample of a foodstuff of known mass,. in the laboratory, you can determine energy content by burning a portion of food and capturing the heat released to a known. The bomb calorimeter is set up as shown in fig.1 above. prepare a table of your results. Prepare the sample in the.
Student instructions and lab report
Food Calorimetry Lab Procedure In this practical, students burn a sample of a foodstuff of known mass,. The caloric content of a food item will be determined using “soda can” calorimetry, where the heat lost through. in the laboratory, you can determine energy content by burning a portion of food and capturing the heat released to a known. The bomb calorimeter is set up as shown in fig.1 above. In this activity, you will burn a piece of food and. prepare a table of your results. in order to obtain caloric content in food, food scientists perform an indirect measure of the amount of heat generated from the burning or. Prepare the sample in the. food calorimetry allows us to determine the number of calories per gram of food. try this class experiment to investigate how much energy different foods contain. Determine the number of kilocalories (food calories) released by the burning food sample. In this practical, students burn a sample of a foodstuff of known mass,.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Food Calorimetry Lab Procedure In this practical, students burn a sample of a foodstuff of known mass,. food calorimetry allows us to determine the number of calories per gram of food. in the laboratory, you can determine energy content by burning a portion of food and capturing the heat released to a known. In this activity, you will burn a piece of. Food Calorimetry Lab Procedure.
From www.animalia-life.club
Food Calorimeter Food Calorimetry Lab Procedure Prepare the sample in the. The bomb calorimeter is set up as shown in fig.1 above. Determine the number of kilocalories (food calories) released by the burning food sample. in order to obtain caloric content in food, food scientists perform an indirect measure of the amount of heat generated from the burning or. try this class experiment to. Food Calorimetry Lab Procedure.
From www.animalia-life.club
Food Calorimeter Food Calorimetry Lab Procedure In this activity, you will burn a piece of food and. Determine the number of kilocalories (food calories) released by the burning food sample. food calorimetry allows us to determine the number of calories per gram of food. The caloric content of a food item will be determined using “soda can” calorimetry, where the heat lost through. in. Food Calorimetry Lab Procedure.
From www.slideshare.net
Calorimetry Lab Food Calorimetry Lab Procedure food calorimetry allows us to determine the number of calories per gram of food. in the laboratory, you can determine energy content by burning a portion of food and capturing the heat released to a known. try this class experiment to investigate how much energy different foods contain. In this practical, students burn a sample of a. Food Calorimetry Lab Procedure.
From www.animalia-life.club
Food Calorimeter Food Calorimetry Lab Procedure In this practical, students burn a sample of a foodstuff of known mass,. food calorimetry allows us to determine the number of calories per gram of food. Determine the number of kilocalories (food calories) released by the burning food sample. In this activity, you will burn a piece of food and. prepare a table of your results. . Food Calorimetry Lab Procedure.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Food Calorimetry Lab Procedure Determine the number of kilocalories (food calories) released by the burning food sample. in order to obtain caloric content in food, food scientists perform an indirect measure of the amount of heat generated from the burning or. food calorimetry allows us to determine the number of calories per gram of food. try this class experiment to investigate. Food Calorimetry Lab Procedure.
From studylib.net
Calorimetry Lab Food Calorimetry Lab Procedure The caloric content of a food item will be determined using “soda can” calorimetry, where the heat lost through. in the laboratory, you can determine energy content by burning a portion of food and capturing the heat released to a known. In this activity, you will burn a piece of food and. In this practical, students burn a sample. Food Calorimetry Lab Procedure.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Food Calorimetry Lab Procedure The bomb calorimeter is set up as shown in fig.1 above. The caloric content of a food item will be determined using “soda can” calorimetry, where the heat lost through. In this practical, students burn a sample of a foodstuff of known mass,. in order to obtain caloric content in food, food scientists perform an indirect measure of the. Food Calorimetry Lab Procedure.
From dxotpxhft.blob.core.windows.net
Food Calorimetry Lab High School at Angelina Britton blog Food Calorimetry Lab Procedure Prepare the sample in the. try this class experiment to investigate how much energy different foods contain. in order to obtain caloric content in food, food scientists perform an indirect measure of the amount of heat generated from the burning or. In this practical, students burn a sample of a foodstuff of known mass,. The caloric content of. Food Calorimetry Lab Procedure.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Food Calorimetry Lab Procedure In this activity, you will burn a piece of food and. prepare a table of your results. Prepare the sample in the. food calorimetry allows us to determine the number of calories per gram of food. in order to obtain caloric content in food, food scientists perform an indirect measure of the amount of heat generated from. Food Calorimetry Lab Procedure.
From kaffee.50webs.com
Lab Calorimetry Food Calorimetry Lab Procedure food calorimetry allows us to determine the number of calories per gram of food. try this class experiment to investigate how much energy different foods contain. Prepare the sample in the. in the laboratory, you can determine energy content by burning a portion of food and capturing the heat released to a known. The caloric content of. Food Calorimetry Lab Procedure.
From www.chegg.com
Solved Lab 6 Food Calorimetry Lab PreLaboratory Food Calorimetry Lab Procedure In this activity, you will burn a piece of food and. The bomb calorimeter is set up as shown in fig.1 above. in order to obtain caloric content in food, food scientists perform an indirect measure of the amount of heat generated from the burning or. in the laboratory, you can determine energy content by burning a portion. Food Calorimetry Lab Procedure.
From dxotpxhft.blob.core.windows.net
Food Calorimetry Lab High School at Angelina Britton blog Food Calorimetry Lab Procedure The bomb calorimeter is set up as shown in fig.1 above. In this activity, you will burn a piece of food and. food calorimetry allows us to determine the number of calories per gram of food. prepare a table of your results. Determine the number of kilocalories (food calories) released by the burning food sample. in the. Food Calorimetry Lab Procedure.
From www.animalia-life.club
Food Calorimeter Food Calorimetry Lab Procedure food calorimetry allows us to determine the number of calories per gram of food. Determine the number of kilocalories (food calories) released by the burning food sample. The caloric content of a food item will be determined using “soda can” calorimetry, where the heat lost through. In this practical, students burn a sample of a foodstuff of known mass,.. Food Calorimetry Lab Procedure.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Food Calorimetry Lab Procedure Prepare the sample in the. The bomb calorimeter is set up as shown in fig.1 above. Determine the number of kilocalories (food calories) released by the burning food sample. try this class experiment to investigate how much energy different foods contain. In this activity, you will burn a piece of food and. in order to obtain caloric content. Food Calorimetry Lab Procedure.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Food Calorimetry Lab Procedure in the laboratory, you can determine energy content by burning a portion of food and capturing the heat released to a known. The caloric content of a food item will be determined using “soda can” calorimetry, where the heat lost through. food calorimetry allows us to determine the number of calories per gram of food. In this activity,. Food Calorimetry Lab Procedure.
From dxotpxhft.blob.core.windows.net
Food Calorimetry Lab High School at Angelina Britton blog Food Calorimetry Lab Procedure prepare a table of your results. Prepare the sample in the. The bomb calorimeter is set up as shown in fig.1 above. In this activity, you will burn a piece of food and. In this practical, students burn a sample of a foodstuff of known mass,. in order to obtain caloric content in food, food scientists perform an. Food Calorimetry Lab Procedure.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Food Calorimetry Lab Procedure Determine the number of kilocalories (food calories) released by the burning food sample. prepare a table of your results. in order to obtain caloric content in food, food scientists perform an indirect measure of the amount of heat generated from the burning or. The caloric content of a food item will be determined using “soda can” calorimetry, where. Food Calorimetry Lab Procedure.